Stanley W. Jacob and Robert Herschler
Department of Surgery • Oregon Health Science University • Portland, Oregon 97201
A wide range of primary pharmacological actions of dimethyl sulfoxide (DMSO) has been documented in laboratory studies: membrane transport, effects on connective tissue, anti-inflammation, nerve blockade (analgesia), bacteriostasis, diuresis, enhancements or reduction of the effectiveness of other drugs, cholinesterase inhibition, nonspecific enhancement of resistance to infection, vasodilation, muscle relaxation, antagonism to platelet aggregation, and influence on serum cholesterol in emperimental hypercholesterolemia. This substance induces differntiation and function of leukemic and other malignant cells. DMSO also has prophylactic radioprotective properties and cryoprotective actions. It protects against ischemic injury. (1986 Academic Press, Inc.)
The pharmacologic actions of dimethyl sulfoxide (DMSO) have stimulated much research. The purpose of this report is to summarize current concepts in this area.
When the theorectical basis of DMSO action is described, we can list literally dozens of primary pharmacologic actions. This relatively brief summary will touch on only a few:
(A) membrane penetration
(B) membrane transport
(C) effects on connective tissue
(D) anti-inflamation
(E) nerve blockade (analgesia)
(F) bacteriostasis
(G) diuresis
(H) enhancement or reduction of effectiveness of other drugs
(I) cholinsterase inhibition
(J) nonspecific enhancement of resistance of infection
(K) vasodilation
(L) muscle relaxation
(M) enhancement of cell differentiation and function
(N) antagonism to platelet aggregation
(O) influence on serum cholesterol in experimental hypercholesterolemia
(P) radio-protective and cryoprotective actions
(Q) protection against ischemic injury
A. Membrane Penetration
DMSO readily crosses most tissue membranes of lower animals and man.
Employing [35S] DMSO, Kolb et al,59 evaluated the absorption and distribution of DMSO in lower animals and man. Ten minutes after the cutaneous application in the rat, radioactivity was measured in the blood. In man radioactivity appeared in the blood 5 minutes after cutaneous application. One hour after application of DMSO to the skin, radioactivity could be detected in the bones.
Denko22 and his associates applied 35S-labeled DMSO to the skin of rats. Within 2 hour a wide range of radioactivity was distributed in all organs studied. The highest values occurred in decreasing order in the following soft tissues; spleen, stomach, lung, vitreous humor, thymus, brain, kidney, sclera, colon, heart, skeletal muscle, skin, liver, aorta, adrenal, lens of eye, and cartilage.
Rammler and Zaffaroni80 have reviewed the chemical properties of DMSO and suggested that the rapid movement of this molecule through the skin, a protein barrier, depends on a reversible configurational change of the protein occurring when DMSO substitutes for water.
B. Membrane Transport
Nonionized molecules of low molecular wight are transported through the skin with DMSO. Substance of high molecular weight such as insulin do not pass through the skin to any significant extent. Studies in our laboratory have revealed that a 90% concentration of DMSO is optimal for the passage of morphine sulfate dissoved in DMSO.77 It would have been expected that 100% would provide better transport than 90%, and the reason for an optimal effect at 90% DMSO remains unexplained. It is of course well known that 70% alcohol has a higher phenol:water partition coefficient than 100% alcohol.
Elfbaum and Laden27 conducted an in vitro skin penetration study employing guinea pig skin as the membrane. They concluded that the passage of picrate ion through this membrane in the presence of DMSO was a passive diffusion process which adhered to Fick's first law of diffusion. It is demonstrated by diffusion and isotope studies that the absolute rate constant for the penetration of DMSO was approximately 100 times greater than that for the picrate ion. Thus, the two substances were transferred through the skin independently of each other. The exact mechanisms involved in the membrane penetrant action of DMSO have yet to be elucidated.
Studies on membrane penetration and carrier effect have been carrier effect have been carried out in agriculture, basic biology, animals, and man. In field tests with severely diseased fruit, Keil55 demonstrated that oxytetracycline satisfactorily controlled bacterial spot in peaches. Control was significantly enhanced by adding DMSO to the antibiotic spray. DMSO was applied to 0.25 and 0.5% with 66 ppm of oxytetracycline. This application gave control of the disease similar to that produced alone by 132 ppm of oxytetracycline and suggested the possibility of diluting the high-priced antibiotic with relatively inexpensive DMSO. There is no good evidence in animals that 0.5% DMSO has significant carrier effects. It could well be that Keil's results were attributable to a carrier effect, but the possibility should always be considered that when DMSO is combined with another substance a new compound results which can then exert a greater or lesser influence on a given process.
Leonard63 studied different concentrations of several water-soluable iron sources applied as foliage sprays to orange and grapefruit trees whose leaves showed visible signs of iron deficiency. The application of iron in DMSO as a spray was followed by a rapid and extensive greening of the leaves, with a higher concentration of chlorophyll.
Amstey and Parkman2 evaluated the influence of DMSO on the infectivity of viral nucleic acid, an indication of its transmembrane transport. It was found that DMSO enhanced polio RNA infectivity in kidney cells from monkeys. Enhancement occurred with all DMSO concentrations from 5 to 80% and was optimal at 40% DMSO, with a 20-minute absorption period at room temperature. A significant percentage of nucleic acid infection was absorbed within the first 2 minutes.
Cochran and his associates14 concluded that concentrations of DMSO below 20% did no influence the infectivity of tobacco mosaic virus (TMV) or the viral RNA. With concentrations between 20 and 60% the infectivity of TMV and TMV RNA varied inversely with the DMSO concentration.
Nadel and co-workers72 suggested that DMSO enhanced the penetration of the infectious agent in experimental leukemia of gunea pigs. Previously Schreck et al.97 had demonstrated that DMSO was more toxic in vitro to lymphocytic leukemia than to lymphocytes from normal patients.
Djan and Gunberg24 studied the percutaneous absorption of 17-estradiol dissolved in DMSO in the immature female rat. These steroids were given in aqueous solutions subcutaneously or were applied topically in DMSO. Vaginal and uterine weight increases resulting from estrogen in DMSO administered topically were comparable to results obtained in animals in which the drugs were administered in pure form subcutaneously.
Smith102 reported that a mixture of DMSO and diptheria toxoid applied frequently to the backs of rabbits causes a reduction of the inflammation produced by the Shick test, indicating that a partial immunity of diphtheria has been produced.
Finney and his associates29 studied the influence of DMSO and DMSO-hydrogen peroxide on the pig myocardium after acute coronary ligation with subsequent myocardial infaction. The addition of DMSO to a hydrogen peroxide perfusion system fascilitated the difffusion of oxygen into the ischemic myocardium.
Maddock et al.66 designed experiments to determine the usefulness of DMSO as a carrier for antitumor agents. The agents were dissoved in 85-100% concentrations of DMSO. One of the tumors studied was the L1210 leukemia. Survival time without treatment was appoximately 8 days. The standard method of employing Cytoxan intraperitoneally produced a survival time of 15.5 days. When Cytoxan was applied topically in water, the survival time was 12.6 days, and topical Cytoxan dissolved in DMSO resulted in survival time of 15.3 days.
Spruance recently studied DMSO as a vehicle for topical antiviral agents, concluding that the penetration of acyclovir (ACV) through guinea pigs skin in vitro was markedly greater with DMSO than when ployethylene glycol (PEG) was the vehicle. When 5% ACV in DMSO was compared with 5% ACV in PEG in the treatmental herpes infection in the guinea pig, ACV DMSO was more effective.103
The possibility of altering the blood-brain diffusion barrrier with DMSO needs additional exploration. Brink and Stein10 employed [14C]pemoline dissolved in DMSO and injected intraperitoneally into rats. It was found in larger amounts in the brain than was a similar dose given in 0.3% tragacanth suspension. The authors postulated that DMSO resulted in a partial breakdown of the blood-brain diffusion barrier in vitro.
There is conflicting evidence as to whether dimethyl sulfoxide can reversibly open the blood-brain barrier and augment brain uptake of water-soluable compounds, including anticancer agents. To investigate this, 125[-Human serum albumin, horse-radish peroxidase, or the anticancer drug melphalan was administered iv to rats or mice, either alone or in combination with DMSO. DMSO administration did not significantly increase the brain uptake of any of the compounds as compared to control uptakes. These results do not support prior reports that DMSO increases the permeability of water-soluable agents across the blood-brain barrier.43
Maibach and Feldmann67 studied the percutaneous penetration of hydrocortisone and testosterone in DMSO. The authors concluded that there was a threefold increase in dermal penetration by these steroids when they were dissolved in DMSO.
Sulzberger and his co-workers107 evaluated the penetration of DMSO into human skin employing methylene blue, iodine, and iron dyes as visual tracers. Biopsies showed that the stratum corneum was completely stained with each tracer applied to the skin surface in DMSO. There was little or no staining below this layer. The authors concluded that DMSO carried substances rapidly and deeply into the horny layer and suggested the usefulness of DMSO as a vehicle for therapeutic agents in inflammatory dermatoses and superficial skin infections such as pyodermas.
Perliman and Wolfe76 demonstrated that allergens of low molecular weight such as penicillin G potassium, mixed in 90% DMSO, were readily carried through intact human skin. Allergens having molecular weights of 3000 or more dissolved in DMSO did not penetrate human skin in these studies. On the other hand, Smith and Hegre101 had previously recorded that antibodies to bovine serum albumin developed when a mixture of DMSO and bovine serum albumin was applied to the skin of rabbits.
Turco and Canada112 have studied the influence of DMSO on lowering electrical skin resistance in man, In combination with 9% sodium chloride in distilled water, 40% DMSO decreased resistance by 100%. It was postulated that DMSO in combination with electrolytes reduced the electrical resistance of the skin by facilitating the absorption of these electrolytes while it was itself being absorbed.
DMSO in some instances will carry substances such as hydrocortisone or hexachlorophene into the deeper layers of the stratum corneum, producing a reservoir.104 This reservoir remains for 16 days and resists depletion by washing of the skin surface with soap, water, or alcohol.105
C. Effect on Collagen
Mayer and associates69 compared the effects of DMSO, DMSO with cortisone acetate, cortisone acetate alone, and saline solutions on the incidence of adhesions following vigorous serosal abrasions of the terminal ileum of Wistar rats. Their technique had developed adhesions in 100% of control animals in 35 days. The treatments were administered daily as postoperative intraperitoneal injections for 35 days. The incidence of adhesions in different groups was DMSO alone: 20%, DMSO-cortisone: 80%, cortisone alone: 100%, saline solution: 100%.
It has been observed in serial biopsy specimens taken from the skin of patients with scleroderma that there is a dissolution of collagen, the elastic fibers remaining intact.93 Gries et al.44 studied rabbit skin before and after 24 hour in vitro exposure to 100% DMSO. After immersion in DMSO the collagen fraction extractable with neutral salt solution was significantly decreased. The authors recorded that topical DMSO in man exerted a significant effect on the pathological deposition of collagen in human postirradiation subcutaneous fibrosis but did not appear to change the equilibrium of collagen metabolism in normal tissue. Urinary hydroxyproline levels are increased in scleroderma patients treated with topical DMSO.93 Keloids biopsied in man before and after DMSO therapy show histological improvement toward normalcy.28
D. Anti-Inflammation
Berliner and Ruhmann7 found that DMSO inhibited fibroblastic proliferation in vitro. Ashley et al.3 reported that DMSO was ineffective in edema following thermal burns of the limbs of rabbits. Formanek and Kovak31 showed that topically applied DMSO inhibited traumatic edema induced by intrapedal injection of autologous blood in the leg of a rat.
DMSO showed no anti-inflammatory effect when studied in experimental effect when studied in experimental inflammation induced in the rabbit eye by mustard oil in the rat ear by croton oil.79
Gorog and Kovacs40 demonstrated that DMSO exerted minimal anti-inflammation effects on edema induced by carrageenan. These authors also studied the anti-inflammatory potential of DMSO in adjuvant-induced polyarthritis of rats. Topical DMSO showed potent anti-inflammatory properties in this model. Gorog and Kovacs41 have also studied the anti-inflammatory activity of topical DMSO, in contact dermatitis, allergic eczema, and calcification of the skin of thr rat, using 70% DMSO to treat the experimental inflammation. All these reactions were significantly inhibited.
The study of Weissmann et al.114 deserves mention in discussing the anti-inflammatory effects of DMSO. Lysosomes can be stabilized against a variety of injurious agents by cortisone, and the concentration of the agent necessary to stabilize lysosomes is reduced 10- to 1000-fold by DMSO. The possibility was suggested that DMSO might render steroids more available to their targets within tissues (membranes of cells or their organelles).
Suckert106 has demonstrated anti-inflammatory effects with intra-articular DMSO in rabbits following the creation of experimental [croton oil] arthritis.
E. Nerve Blockade (Analgesia)
Immersion of the sciatic nerve in 6% DMSO decreases the conduction velocity by 40%. This effect is totally reversed by washing the nerve in a buffer for 1 hour.89 Shealy99 studied peripheral small fiber after-discharge in the cat. Concentrations of 5-10% DMSO eliminated the activity of C fibers with 1 minute: activity of the fibers returned after the DMSO was washed away.
DMSO injected subcutaneously in 10% concentration into cats produced a total loss of the central pain response. Two milliliters of 50% DMSO injected into the cerebrospinal fluid led to total anesthesia of the animal for 30 minutes. Complete recovery of the animal occurred without apparent ill effect.100
Haigler concluded that DMSO is a drug that produced analgesia by acting both locally and systemically. The analgesia appeared to be unrelated to that produced by morphine although the two appear to be a comparable magnitude. DMSO had a longer duration of action than morphine, 6 hr vs 2 hr, respectively.45
F. Bacteriostasis
DMSO exerts a marked inhibitory effect on a wide range of bacteria and fungi including at least one parasite, at concentrations (30-50%) likely to be encountered in antimicrobial testing programs in industry.6
DMSO at 80% concentration inactivated viruses tested by Chan and Gadenbusch. These viruses included four RNA viruses, influenza A virus, influenza A-2 virus, Newcastle disease virus, Semliki Forest virus, and DNA viruses.12
Seibert and co-worker98 studied the highly pleomorphic bacteria regularly isolated from human tumors and leukemic blood. DMSO in 12.5-25% concentration caused complete inhibition of growth in vitro of 27 such organisms without affecting the intact blood cells.
Among the intriguing possibilities for the use of DMSO is its ability to alter bacterial resistance. Pottz and associates78 presented evidence that the tubercle bacillus, resistant to 2000Ęg of treptomycin or isoniazide, became sensitive to 10Ęg of either drug after pretreatment with 0.5-5% DMSO.
Kamiya et al.54 found that 5% DMSO restored and increased the sensitivity of antibiotic-resistant strains of bacteria. In particular, the sensitivity of all four strains of Pseudomonas to colistin was restored when the medium contained 5% DMSO. The authors recorded that antibiotics not effective against certain bacteria, such as penicillin to E. coli, showed growth inhibitory effects when the medium contained DMSO.
Ghajar and Harmon35 studied the influence of DMSO on the permeability of Staphylococcus aureau, demonstrating that DMSO increased the oxygen uptake but reduced the rate of glycine transport. They could not define the exact mechanism by which DMSO produced its bacteriostatic effect.
Gillchriest and Nelson37 have suggested that bacteriostasis from DMSO occurs due to a loss of RNA conformational structure required for protein synthesis.
G. Diuresis
Formanek and Suckert32 studied the diuretic effects of DMSO administered topically to rats five times daily in a dosage of 0.5 ml of 90% DMSO per animal. The urine volume was increased 10-fold, and with the increase in urine volume, there was an increase in sodium and potassium excretion.
H. Enhancement or Reduction of Concomitant Drug Action
Rosen and associates84 employed aqueous DMSO to alter the LD50 in rats and mice when oral quaternary ammonium salts were used as test compounds. In rats, the toxicity of pentolinium tartrate and hexamethonium bitartrate was increased by DMSO, while the toxicity of hexamethonium iodide was decreased.
Male68 has shown that DMSO concentrations of upward to 10% lead to a decided increase in the effectiveness of griseofulvin.
Melville and co-workers70 have studied the potentiating action of DMSO on cardioactive glycosides in cats, including the fact that DMSO potentiates the action of digitoxin. This effect, however, does not appear to involve any change in the rate of uptake (influx) or the rate of loss (efflux) of glycosides in the heart.
I. Cholinesterase
Sams et al.90 studied the effects of DMSO on skeletal, smooth, and cardiac muscle, employing concentrations of 0.6-6%. DMSO strikingly depressed the response of the diaphragm to both direct (muscle) and indirect (nerve) electrical stimulation, and caused spontaneous skeletal muscle fasciculations. DMSO increased the response of the smooth muscle of the stomach to both muscle and nerve stimulations. The vagal threshold was lowered 50% by 6% DMSO. Cholinesterase inhibition could reasonably explain fasciculations of skeletal muscle, increased tone of smooth muscle, and the lower vagal threshold observed in these experiments. In vitro assays show that 0.8-8% DMSO inhibits bovine erythrocyte cholinesterase 16-18%.
J. Nonspecific Enhancement of Resistance
In a study of antigen-antibody reactions, Reattig81 showed that DMSO did not disturb the immune response. In fact, the oral administration of DMSO to mice for 10 days prior to an oral infection with murine typhus produced a leukocytosis and enhanced resistance to the bacterial infection.
K. Vasodilation
Adamson and his co-workers1 applied DMSO to a 3-1 pedicle flap raised on the back of rats. The anticipated slough was decreased by 70%. The authors suggested that the primary action of DMSO on pedicle flap circulation was to provoke a histamine-like reponse. Roth87 has also evaluated the effects of DMSO on pedicle flap blood flow and survival, concluding that DMSO does indeed increase pedicle flap survival, but postulating that this increase takes place by some mechanism other than augmentation of perfusion. Kligman56, 57 had previously demonstrated that DMSO possesses potent histamine-liberating properties.
Leon62 has studied the influence of DMSO on experimental myocardial necrosis. DMSO therapy effected a distinct modification with less myocardial fiber necrosis and reduced residual myocardial fibrosis. The author reported that neither myocardial rupture nor aneurysm occured in the group treated with DMSO.
L. Muscle Relaxation
DMSO applied topically to the skin of patients produces electromyographic evidence of muscle relaxation 1 hour after application.8
M. Antagonism to Platelet Aggregation
Deutsch23 has presented experimental data showing that 5% DMSO lessons the adhesiveness of blood platelets in vitro. Gorog39 has shown that DMSO is a good antagonist to platelet aggregation as well as thrombus formation in vivo. Gorog evaluated this in the hamster cheek pouch model.
N. Enhancement of Cell Differentiation and Function
It has been shown that dimethyl sulfoxide induces differentiation and function of leukemic cells of mouse 11, 33, 46, 65, 92, 115, rat,58 and human.9, 15, 16, 34, 109 DMSO was also found to stimulate albumin production in malignantly transformed hepatocytes of mouse and rat49 and to affect the membrane-associated antigen, enzymes, and glycoproteins in human rectal adenocarcinoma cells.111 Hydrocortisone-induced keratinization of chick embryo cells74 and adriamcycin-induced necrosis of rat skin108 were inhibited by DMSO.
Furthermore, modification by DMSO of the function of normal cells has been reported. DMSO stimulates cyclic AMP accumulation and lipolysis and decreases insulin-stimulated glucose oxidation in free white fat cells of [the] rat. It also enhances heme synthesis in quail embryo yolk sac cells.110
Leukemic blasts can be induced by external chemical agents to mature to neutrophils, monocytes, or RBCs. The phenotype of leukemic cells thus results from both internal genetic aberrations and the response of leukemic cells to their external environment. When human myeloid leukemia cells are exposed in vitro to a variety of agents (e.g.vitamin A or dimenthyl sulfoxide) the blasts lose their proliferative potential, the expression of oncogene products is sharply decreased, and after 5 days the leukemic cells become morphologically mature and functional neutrophils. Some patients with myeloid leukemias have responded to therapy designed to induce maturation in vivo. The induced maturation of leukemic cells is a new therapeutic tactic-alternative to cytotoxic drug therapy-wherein leukemic cells are destroyed by transforming them into neutrophils.86
O. Influence on Serum Cholesterol in Experimental Hypercholesterolemia
Rabbits given a high cholesterol diet with 1% DMSO showed one-half as much hypercholesterolemia as control animals.48
P. Radioprotective and Cryoprotective Actions
M.J. Ashwood-Smith has written a comprehensive review of these actions.4
Q. Protection against Ischemic Injury
De la Torre has advanced a scheme based on both investigated and theoretical actions of DMSO on the biochemical events generated after an ischemic injury. He previously proposed this hypothetical model to help conceptualize how DMSO, or similar drugs, mights affect the pathochemical balance that results in lack of tissue perfusion following trauma.19
The biochemical and vascular responses to injury appear to have a cause and effect relationship that can be integrated in terms of substances that either increase or decrease blood flow. The substance's effect can be physical, i.e. reduce or increase the vessel lumen obstruction, or chemical, i.e. reduce or increase the vessel lumen diameter (vasoconstriction/vasodilation).
Platelets, for example, can induce both conditions. Obstruction of the vessel lumen can result from platelet adhesion (platelet buildup in damaged vessel lining) or platelet aggregation. Platelet damage moreover can cause vasoconstriction or vasospasm by liberating vasoactive substances locally with the blood vessel or perivascularly, if penetrating damage to the vessel has occurred. There are two storage sites within platelets that contain most of these vasoactive substances. The alpha granules contain fibrinogen, while the dense bodies store ATP, ADP, serotonin, and calcium, which can be secreted by the platelet into the circulation by a canalicular system.5 Thromboxane A2 has also been shown to be manufactured in the microsomal fraction of animal and human platelets.73 All these vasoactive substances (with the exception of ATP) can cause significant reduction of blood flow by physical or chemical reactivity on the vasculature.
DMSO can antagonize a number of these vasoactive substances released by the platelets, which could consequently induce vasoconstriction, vasospasm, or obstruction of vessel lumen. For example, a study has shown that DMSO can inhibit ADP and thrombin-induced platelet aggregation in vitro.95 It may presumable do this by increasing the evels of cAMP (a strong platelet deaggregator) through inhibition of its degradative enzyme, phosphodiesterase.26, 51 DMSO is reported to deaggregate platelets in vivo following experimental cerebral ischemia.26, 51 This effect may be fundamental in view of the finding that cerebral ischemia produces transient platelet abnormalities that may promote microvascular aggregation formation and extend the area of ischemic injury.25
The biochemical picture is further complicated by the possible activity of DMSO on other vasoactive substances secreted by the platelets during injury or ischemia. For example, the release of calcium from cells from cells or platelets and its effect on arteriolar-wall muscle spasm may be antagonized by circulating DMSO.13, 88 Collagen-induced platelet release may also be blocked by DMSO.44, 94
The following effects of DMSO are likely to be involved in its ability to protect against ischemic injury.
Little is known about the actions of DMSO on the prostanoids (PG/TX). Studies have reported that DMSO can increase the synthesis of PGE1, a moderate vasodilator.61. PGE1 can reduce platelet aggregation by increasing cAMP levels and also inhibit the calcium-induced release of noradrenalin in nerve terminals, an affect that may antagonize vasoconstriction and reduction of cerebral blood flow.53
DMSO, it will be recalled, also has a direct effect on cAMP. It increases cAMP presumably by inhibiting phosphodiesterase,113 although an indirect action on PGI2-induced elevation of platelet cAMP by DMSO should not be ruled out. Any process that increases platelet cAMP will exert strong platelet deaggregation.
It has also been reported that DMSO can block PFG2 receptors and reduce PFE2 synthesis.82 Both these compounds can cause moderate platelet aggregation and PFG2 is known to induce vasoconstriction.60 The effects of DMSO on thromboxane synthesis are unknown. It could, however, inhibit TXA2, biosynthesis in much the same way as hydralazine or dipyridamole42 since it shares a number of similar properties with these agents: specifically, their increase of cAMP levels.
The ability of DMSO to protect cell membrane integrity in various injury models is well documented.38, 64, 91, 114
Cell membrane preservation by DMSO might help explain its ability to improve cerebral and spinal cord blood flow after injury.18 DMSO could be preventing impairment of cerebrovascular endothelial surfaces where PGI2 is elaborated and where platelets can accumulate following injury. The effects of DMSO may be two-fold: reduction of platelet adhesion by collagen,44 and reduction of platelet adhesion by protecting the vascular endothelium and ensuring PGI2 release.
Although many hormones, chemical transmitters, peptides, and numerous enzymes can be found in mammalian circulation at any given time, it is the hydrozyl radicals that have drawn attention by playing an important role in the pathogenesis of ischemia.21, 30 Free radicals can be elaborated by peroxidation of cellular membrane-bound lipids where oxygen delivery is not totally abolished, as in ischemia and hypoxia, or when oxygen is resupplied after an ischemic episode.83
One of the significant sites where hydroxyl radicals can form following ischemia is in mitochondria. DMSO is known to be an effective hydroxyl radical scavenger.4, 20, 75 Since it has been shown that DMSO can improve mitochondrial oxidative phosphorylation, it has been suggested that DMSO may act to neutralize the cytotoxic effects of hydroxyl radicals in mitochondria themselves.96 Oxidative phosphorylation is one of the primary biochemical activities to be negatively affected following ischemic injury. DMSO has also been reported to reduce ATPase activity in submitochondrial particles,17, 36 an effect that can lower oxygen utilization during cellular ischemia.
It has been proposed that DMSO may reduce the utilization of oxygen by an inhibiting effect on mitochondrial function. In one experiment the energy loss due to inhibition of oxidative activity after brain tissue was perfused with DMSO was compensated for by an increase in glycolysis.36
It seems probable that the neutralizing action of DMSO on hydroxyl radical damage following injury could diminish the negative outcome of ischemia. However the formation of hydroxyl radicals is dependent on time and oxygen availability, but the development of ischemia is immediate and its reversal may depend on more prevalent subsystems such as the PG/TX and platelet interactions. Maintaining the balance of these subsystems appears more critical in predisposing the outcome of cerebral ischemia.
Another interesting effect of DMSO is on calcium. When isolated rat hearts are perfused with calcium-free solution followed by reperfusion with a calcium-containing solution, a massive release of creatine kinase (indicating cardiac injury) is observed. This creatine kinase level increase is accompanied by electrocardiographic (EKG) changes and ultrastructural cell damage.50 DMSO has been reported to significantly reduce the release of creatine kinase and prevent EKG and ultrastructural changes if it is present during reperfusion of the isolated rat heart with a calcium-containing solution.88 Moreover, examination of the heart tissue by electron microscopy showed that DMSO-treated preparations lacked the mitochondrial swelling and contraction band formation otherwise induced by the reentry of calcium.88 These findings are supported by another investigation showing that DMSO can block calcium-induced degeneration of isolated myocardial cells.13 This protective effect by DMSO on myocardial tissue may be critical during ischemic myocardial infarction when evolutionary EKG changes, serum creates kinase levels are elevated, and myocardial necrosis can develop rapidly.
DMSO2 is not an effective cryoprotective agent; however, Herschler47 has recorded that DMSO (dimethyl sulfone) is a natural source of biotransformable sulfur in plants and lower animals. Jacob and Herschler have reported a number of unique properties possessed by DMSO.52 Since DMSO is oxidized to DMSO2 in vivo, scientists should include DMSO as a control in basic biologic studies on DMSO in plants and animals.
Footnotes
(a) Although the abbreviation "Me2SO" has been recommended for chemists by the IUPAC, the abbreviation for dimethyl sulfoxide most familiar to those concerned with its medicinal uses is "DMSO." Consequently, this generic pharmacological name for dimethyl sulfoxide will be employed throughout this paper.
(b) Supported in part by a grant from The Ronald J. Purer Foundation. Presented at the Symposium Biological Effects of Cryoprotective Agents at the Cryobiology Meeting, June 1985, Madison, Wis.
(c) Stanley W. Jacob, MD, Gerlinger Associate Professor of Surgery and Surgical Research.
References
- Adamson, J. E., Crawford, H. H., and Horton, C.E. The action of dimenthyl sulfoxide on the experimental pedicle flap. Surg. Forum. 17:491-492 (1966).
- Amstey, M.S., and Parkman, P.D. Enhancement of polio RNA infectivity by dimenthyl sulfoxie. Proc. Soc. Exp. Biol. Med. 123:438-442 (1966)
- Ashley, F.L., Johnson, A.N., McConnell, D.V., Galloway, D.V., Machida, R.C., and Sterling, H.E. Dimethyl Sulfoxide and burn edema. Ann. N.Y. Acad. Sci. 141: 463-464 (1967).
- Ashwood-Smith, M.J. Current concepts concerning radioprotective and cryroprotetive properties or dimethyl sulfoxide in cellular systems. Ann. N.Y. Acad. Sci. 141: 41-62 (1967).
- Baldini, M.G., and Myers, T.J. One more variety of storage pool disease. J. Amer Med. Assoc. 244: 173-175 (1980).
- Basch, H., and Gadebusch, H.H. In vitro antimicrobial activity of dimethyl sulfoxide. Appl. Microbiol. 16: 1953-1954 (1968).
- Berliner,D.L., and Ruhmann, A.G. The influence of dimethyl sulfoxide on fibroblastic proliferation. Ann. N.Y. Acad. Sci. 141: 159-164 (1964).
- Birkmayer, W., Danielczyk, W., and Werner, H. DMSO bei spondylogenen neuropathien. In "DMSO Symposium, Vienna, 1966" (G. Laudahn and K. Gertich, Eds.) pp. 134-136 Saladruck, Berlin (1966).
- Bonder, R.W., Siegel, M.I., McConnell, R.T., and Cuatrecasesk P. The appearance of phospholipase and cyclo-oxygenase activities in the human promyelocytic leukemia cell line HL-60 during dimethyl sulfoxide-induced differentiation. Biochem. Biophys. Res Commun. 98: 614-620 (1981).
- Brink, J.J., and Stein. D.G. Pemoline levels in brain-enhancement by dimethyl sulfoxide. Science (Washington, D.C.) 158: 1479-1480 (1967).
- Brown, A.E., Schwartz, E.L., Dreyer, R.N., and Satrorelli, A.C. Synthesis of sialoglycoconjugates during dimethyl sulfoxide-induced erythrodifferentiation of Friend Leukemia cells. Biochem. Biophys. Acta 717: 217-225 (1982).
- Chan, J.C., and Gadebusch, H.H. Virucidal properties of dimethyl sulfoxide. Appl. Microbiol. 16: 1625-1626 (1968).
- Clark, M.G., Gannon, B.J., Bodkin, N., Patten, G.S., and Berry, M.N. An improved procedure for high-yield preparation of intact beating heart cells from adult rat: Biochemical and moronologic study. J. Mol. Cell. Cariol 10: 1101-1121 (1978).
- Cohran, G.W., Dhaliwal, A.S., Forghani, B. Chideste, J.L., Dhaliwal, G.K., and Lambron, C.R. Action of dimethyl sulfoxide on tobacco mosaic virus. Phytopathology 57, 97 (1967). (abstract).
- Collins, S.J., Ruscetti, F.W., Gallagher, R.E., and Gallo, R.C. Terminal differentiation of human promyelocytic leukemia cells induced in dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA 75: 2458-2462 (1978).
- Collins, S.J., Ruscetti, F.W., Gallagher, R.E., and Gallo, R.C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentation by dimethyl sulfoxide. J. Exp. Med. 149: 969-974 (1979).
- Conover, T.E. Influence of nonionic organic solutes on various reactions of energy conservation and utilization. Ann. N.Y. Acad. Sci. 243: 24-37 (1975).
- De la Torre, J.C. Spinal Cord Injury: Review of basic and applied research. Spine 6. 315-335 (1981).
- De la Torre, J.C., Surgeon, J. W., Hill, P.K., and Khan, T. DMSO in the treatment of brain infrction: Basic considerations. In "Arterial Air Embolism and Acute Stroke: Report No. 11/15/77" (J.M. Hallenbeck and L. Greenbaum, Eds.) pp. 138-161. Undersea Medical Society, Bethesda, Md. (1977).
- Del Maestro, R., Thaw, H.H., Bjork, J., Planker, M., and Arfors, K.E. Free radicals as mediators of tissue injury. Acta. Physiol. Scand. Suppl. 492: 91-119 (1980).
- Demonpoulos, H.B., Flamm, E., Pietronigro, D., and Seligman, M.L. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta. Physiol. Scand. Suppl. 492: 91-119 (1980).
- Denko, C.W., Goodman, R.M., Miller, R., and Donovan, T. Distribution of dimthyl sulfoxide-35S in the rat. Ann. N.Y. Acad. Sci. 141: 77084 (1967).
- Deutsch, E., Beeinflussung der Blutgerinnung durch DMSO und Kombinationen mit Heparin, In "DMSO Symposium, Vienna, 1966" (G. Laudahn and K. Gertich, Eds.) pp.144-149. Saladruck, Berlin. 1966.
- Djan, T.I., and Gunber, D.L. Percutaneous absorption of two steriods dissoved in dimethyl surlfoxide in the immature female rat. Ann. N.Y. Acad. Sci. 141: 406-413 (1967).
- Dougherty, J.H., Levy, D.E., and Weksler, B.B. Experimental cerebral ischemia produces platelet aggregates. Neurology 29: 1460-1465 (1979).
- Dujovny, M., Rozano, R., Kossovsky, N., Diaz, F.G., and Segal, R. Antiplatelet effect of dimethyl sulfoxide. barbiturates and methyl prednisoione. Ann. N.Y. Acad. Sci. 441:234-244 (1983).
- Elfbaum, S.G., and Laden K. Effect of dimethyl sulfoxide on percutaneous absorption--a mechanistic study. J. Soc. Cosmet. Chem. 19, 841 (1968) (Abstract).
- Engle, M.F. Indications and contraindications for the use of DMSO in clinical dermatology. Ann. N.Y. Acad. Sci. 141: 638-645 (1967).
- Finney, J.W., Urschel, H.C., Balla, G.A., Race, G.J., Jay, B.E., Pingree, H.P., Dorman, H.L., and Mallams, J.T. Protection of the ischemic heart with DMSO alone or DMSO with hydrogen peroxide. Ann. N.Y. Acad. Sci. 141: 231-241 (1967).
- Flamm, E.S., Demonpoulos, H., Seligman, M., and Ransohoff, J. Free radicals in cerebral ischemica. Stroke 9: 445-447 (1978).
- Formanek, K., and Kovac, W. DMSO bei experimentellen Rattenpfotenodemen. In "DMSO Symposium, Vienna, 1966" (G. Laudahn and K. Gertich, Eds.). pp.18-24. Saladruck. Berlin. 1966.
- Formanek, K., and Suckert, R. Diuretische Wirkung von DMSO. In "DMSO Symposium, Vienna, 1966" (G. Laudahn and K. Gertich, Eds.). pp.21-24. Saladruck. Berlin. 1966.
- Friend, C., Scher, W., Holland, J.G., and Sato. T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: Stimulation of erythroid differentiation by dimethyl sulfoxide. Proc. Natl. Acad. Sci. USA 68: 378-382.(1971).
- Gahmberg, C.G., Nilsson, K., and Anderson, L.C. Specific changes in the surface glycoprotein pattern of human promyelocytic leukemic cell line HL-60 during morphologic and functional differentiation. Proc. Natl. Acad. Sci. USA 76: 4087-4091. (1979).
- Ghajar, B.M., and Harmon, S.A. Effect of dimethyl sulfoxide (DMSO) on permeability of Staphylococcus aurens. Biochem. Biophys. Res. Commun. 32: 940-944 (1968).
- Ghosh, A.K., Ito, T., Ghosh, S., and Sloviter, H.A. Effects of dimethyl sulfoxide on metabolism of isolated perfused rat brain. Biochem Pharmacol. 25: 1115-1117 (1976).
- Gillchriest, W.C., and Nelson, P.L. Protein synthesis in bacterial and mammalian cells. Biophys. J. 9: A-133 (1969).
- Gollan, F. Effect of DMSO and THAM on ionizing radiation in mice. Ann. N.Y. Acad. Sci. 141: 63-64 (1967).
- Gorog, P. Personal communications. May 10, 1969.
- Gorog, P., and Kovacs, I.B. Effect on dimethyl sulfoxide (DMSO) on various experimental inflammations. Curr. Ther. Res. 10: 486-492 (1968).
- Gorog, P., and Kovaces, I.B. Effect of dimethyl sulfoxide (DMSO) on various experimental cutaneous ractions. Pharmacology 67: in press.
- Greenwald, J.E., Wong, K.E., Alexander, M., and Bianchine, J.R. In vitro inhibition of thromboxane biosynthesis by hydralazine. Adv. Prostaglandin Thromboxane Res. 6: 293-295 (1980).
- Greig, N.H., Sweeney, D.J., and Rapoport, S. I. Inability of dimethyl sulfoxide to increase brain uptake of water-soluble compounds: Implications to chemotherapy for brain tumors. Cancer Treat. Rep. 69: 305-12 (1985).
- Gries, G., Bublitz, G., and Lindner. J. The effect of dimethyl sulfoxide on the components of connective tissue (Clinical and experimental investigations). Ann. N.Y. Acad. Sci. 141: 630-637 (1967).
- Haigler, H.J. Comparison of the analgesic effects of dimethyl sulfoxide and morphine. Ann. N.Y. Acad. Sci. 411: 19-27 (1983).
- Hannania, N., Shaool, D., Poncy, C., and Harel, J. New gene expression in dimethyl sulfoxide treated Friend erytholeukemia cells. Exp. Cell Res. 130: 119-126 (1980).
- Herschler, R.J. Unpublished data.
- Herzmann, E. Studies of the effect of dimethyl sulfoxide on experimental hypercholesterolemia in young cocks. Acta. Biol. Med. Ger. 20: 483-487 (1968).
- Higgins, P.J., and Borentreund, E. Enhanced albumin production by malignantly transformed hepatocytes during in vitro exposure to dimethyl sulfoxide. Biochum. Biophys. Acta. 610: 174-180 (1980).
- Holland, C.E., and Olson, R.E. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J. Mol. Cell Cardiol. 7: 917-928 (1975).
- Holtz, G.C., and Davis, R.B. Inhibition of human platelet aggregation in dimethyl sulfoxide, dimethyl acetamidine and sodium glycerophosphate. Proc. Soc. Exp. Biol. Med. 141: 244-248 (1974).
- Jacob, S.W., and Herschler, R. Introductory remarks: Dimethyl sulfoxide after twenty years. Ann. N.Y. Acad. Sci. 441: xiii-xvii (1983).
- Johnson, M. and Ramwell, P.W. Implications of prostaglandins in hematology. In "Prostaglandins and Cyclic AMP" (R.H. Kahn and W.E.M. Lands, Eds.) pp. 275-304. Academic Press, New York, (1974).
- Kamiya, S., Wakao., T., and Nishioka, K. Studies on improvement of eye drops. Bacteriological consideration of DMSO. Jpn. J. Clin. Opthalmol. Rinsho Gank. 20: 143-152 (1966).
- Keil, H. L. Enhanced bacterial sport control on peach when dimethyl sulfoxide is combined with sprays of oxytetracycline. Ann. N.Y. Acad. Sci. 141: 131-138 (1967).
- Kligman, A.M. Topical pharacology and toxicology or dimethyl sulfoxide (DMSO). Part 1. J. Amer. Med. Assoc. 193: 796-804 (1965).
- Kligman. A.M. Topical pharmacology and toxicology of dimethyl sulfoxide (DMSO). Part 2. J. Amer. Med. Assoc. 193: 923-928 (1965).
- Kluge, N., Ostertag, W., Sugiyama, D., Arndt-Jovin, D., Steinheider, G., Furusawa, M., and Dube, S. Dimethyl sulfoxide-induced differentiation and hemoglobin synthesis in tissue culture of rat erythroleukemia cells transformed by 7.12-dimethylbenz(a)anthracene. Proc. Natl. Acad. Sci. 73: 1237-1240 (1976).
- Kolb, K.H., Janicke, G., Kramer, M., Schulze, P.E., and Raspe, G. Absorption, distribution and elimination of labeled dimethyl sulfoxide in man and animals. Ann. N.Y. Acad. Sci. 141: 85095 (1967).
- Lefer, A.M. Role of the prostaglandin-thromboxane system in vascular homeostasis during shock. Circ. Shock 6: 297-303. (1979).
- LeHann, T.R., and Horita, A. Effects of dimethyl sulfoxide (DMSO) on prostaglandin synthetase. Proc. West Pharacol. Soc. 18: 81-82 (1975).
- Leon, A. Personal communication. June 5, 1969.
- Leonard, C.D. Use of dimethyl sulfoxide as a carrier for iron in nutritional foliar sprays applied to citrus. Ann. N.Y. Acad. Sci. 141: 148-158. (1967).
- Lim, R., and Mullan, S. Enhancement of resistance of glial cells by dimethyl sulfoxide against sonic disruption. Ann. N.Y. Sci. 243: 358-361 (1975).
- Lin, C.S. and Lin, M.C. Appearance of late-adrenergic response of adenylate cyclase during the induction of differentiation in cell cultures. Exp. Cell. Res. 112: 339-402. (1979).
- Maddock, C.L., Green, M.N., and Brown, B.L. Topical administration of anti-tumor agents to locally implanted neoplasma. Proc. Amer. A. Cancer Res. 7. 46. (1966) (abstract).
- Maibach, H. I., and Feldmann, R. J. The effect on DMSO of percutaneous penetration of hydrocortisone and testosterone in man. Ann. N.Y. Acad. Sci. 141: 423-427 (1967).
- Male, O. Enhancement of the antimycetic effectiveness of Griseo-Fulvin by dimethyl sulfoxide in vitro. Arch. Klin. Exp. Dermatol. 223: 63-76 (1968).
- Mayer, J.H., III., Anido, H., Almond, C.H., and Seaber., A. Dimethyl sulfoxide in prevention of intestinal adhesions. Arch. Surg. 91: 920-923. (1965).
- Melville, K.I., Klingner, B., and Shister, H.E. Effects of dimethyl sulfoxide (DMSO) on cardiovascular responses to Quabain. Proscillaridin and Digitoxin. Arch. Intern. Pharmacodyn.174: 277-293. (1968).
- Munoz, L.G., Rozario, R.A., Dujovny, M., and Stroth, D. Antiplatelet properties of DMSO and barbiturates in microvessels with scanning electron microscopy. J. Neurosurg. 52: 450 (1980) (Abstract).
- Nadel, E.M., Nobel, J.G., Jr., and Burstein, S. Observations on an effect of ACTH. dexamethasone. and dimethyl sulfoxide (DMSO) on the "out of strain" transplantation and lethality of strain 2 guinea pig leukemia LSC NB to strain 13 and Hartly animals. Cryobiology 5: 254-261 (1969).
- Needleman, P., Moncade, S., Bunting, S., Vane, J.R., Hamber, M., and Samuelsson, B. Identification of any enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature (London) 261: 558-560 (1976).
- Obinata, A., Takata, K., Kawada, M., Hirano, H., and Endo, H. Reversible inhibition by DMSO of hydrocortisone-induced keratinization of chick embryonic skin. Exp. Cell Res. 138: 135-145 (1982).
- Panganamala, R.V., Sharma, H.M., and Heikkila, R.E. Role of hydroxyl radical scavengers, dimethyl sulfoxide, alcohols, and methional in the inhibition of prostaglandin synthesis. Prostaglandins 11: 599-607 (1976).
- Perlman, F., and Wolfe, H.F. Dimethyl Sulfoxide as a penetrant carrier of allergens through intact human skin. J. Allergy 38: 299-307 (1966).
- Phatek, N. Personal communication. June 5. (1969).
- Pottz, G.E., Rampey, H., Jr, and Benjamin, A. Die verwendung von DMSO zur Schellfarbung von Mykobakterien und anderen Mikroorganismen in Abstrichen und Gewbeschnitten. In "DMSO Symposium, Vienna. 1966." (G. Laudahand, K. Gertich, Eds.). pp.40-43. Saladruck. Berlin. 1966.
- Preziosi, P., and Scapgnini, U. Action of DMSO on acut inflammatory reactions. Current Therap. Res. 8: 261-266. (1966).
- Rammier, D.H., and Zaffaroni, A. Biological implications of DMSO based on a review of its chemical properties. Ann. N.Y. Acad. Sci. 141: 13-23 (1967).
- Raettig, H. Die Moglichkeiten des DMSO in der experimentellen immunogie. In "DMSO Symposium, Vienna, 1966" (G. Laudahn and K. Gertich, Eds.) pp. 51-56. Saladruck, Berlin, (1966).
- Rao, C.V. Differential effects of detergents and dimethyl sulfoxide on membrane prostaglandin E, and F, receptors. Life Sci. 20: 2013-2022 (1977).
- Rehncrona, S., Siesjo, B.K., and Smith, D.S. Reversible ischemia of the brain: Biochemical factors influencing restitution. Acta. Physiol. Scand. Suppl. 492: 135-140 (1980).
- Rosen, H., Blumenthal, A., Panacvich, R., and McCallum, J. Dimethyl sulfoxide (DMSO) as a solvent in acute toxicity determinations. Proc. Soc. Exp. Bio. Med. 120: 511-514 (1965).
- Rosenblum, W.I., and El-Sabban, F. Dimethyl sulfoxide and glycerol, nydroxyl radical scavengers, impair platelet aggregation within and eliminate the accompanying vasodilation of injured mouse pial arterioles. Stroke 13: 35-39 (1982).
- Ross, D.W. Leukemic cell maturation. Arch. Pathol. Lab. Med. 109: 309-313 (1985).
- Roth, C.A. Effects of dimethyl sulfoxide on pedicle flap flow and survival. J. Amer. Med. Women's Assoc. 23: 895-898 (1968).
- Ruigrok, T.J.C., DeMoes, D., Slade, A.M., and Nayler, W.G. The effect of dimethyl sulfoxide on the calcium paradox. Amer. J. Pathol. 103: 390-403 (1981).
- Sams, W.M., Jr. The effects of dimethyl sulfoxide on nerve conduction. Ann. N.Y. Acad. Sci. 141: 242-247 (1967).
- Sams, W.M., Carroll, N.V., and Crantz, P.L. Effects of dimethyl sulfoxide on isolated innervated skeletal smooth and cardiac muscle. Proc. Soc. Exp. Biol. Med. 122: 103-107 (1966).
- Sandborn, E.B., Stephens, H., and Bendayan, M. The influence of demethyl sulfoxide on cellular ultrastructure and cytochemistry. Ann. N.Y. Acad. Sci. 243: 122-138 (1975).
- Scher, B.M., Scher, W., Robinson, A., and Waxman, S. DNA ligase and DNase activities in mouse erythroleukemic cells during dimethyl sulfoxide-induced differentiation. Cancer Res. 42: 1300-1306 (1982).
- Scherbel, A.L., McCormack, L.J., and Layle, J.K. Further observations on the effect of dimethyl sulfoxide in patients with generalized scleroderma (progressive systemic sclerosis). Ann. N.Y. Sci. 141: 613-629 (1967).
- Scherbel, A.L., McCormack, L.J., and Poppo, M.J. Alterations of collagen in generalized scleroderma (progressive systemic sclerosis) after treatment with dimethyl sulfoxide. Cleveland Clin. Q. 32: 47-58 (1965).
- Schiffer, C.A., Whitaker, C.l., Schmukler, M., Aisner, J., and Hibert, S.L. The effect of dimethyl sulfoxide on in vitro platelet function. Thromb. Huemostasis 36: 221-229 (1976).
- Schlafer, M., Kane, P.F., and Kirsch, M. Effects of dimethyl sulfoxide on the globally ischemic heart: Possible general relevance to hypothermic organ preservation. Cryobiology. 19: 61-69 (1982).
- Schreck, R., Elrod, L.M., and Batra, K.V. Cytocidal effects of dimethyl sulfoxide on normal leukemic lymphocytes. Ann. N.Y. Acad. Sci. 141: 202-213 (1967).
- Seibert, F.B., Farrelly, F.K., and Shepherd, C.C. DMSO and other combatants against bacteria isolated from leukemia and cancer patients. Ann. N.Y. Acad. Sci. 141: 175-201 (1967).
- Shealy, C.N. The physiological substrate of pain. Headache 6: 101-108 (1966).
- Shealy, C.N. Personal communication. June 5, 1969.
- Smith, R.E., and Hegre, A.M.
- Smith, R.E. The use of dimethyl sulfoxide in allergy and immunology. E.E.N.T. Digest 30: 47-54 (1968).
- Spruance, S.L., McKeough, M.B., and Cardinal, J.R. Dimethyl Sulfoxide as a vehicle for topical antiviral chemotherapy. Ann. N.Y. Acad. Sci. 411: 28-33 (1983).
- Stoughton, R.B. Dimethyl sulfoxide (DMSO) induction of a steroid reservoir in human skin. Arch. Dermatol. 91: 657-660 (1965).
- Stoughton, R.B. Hexachlorophene deposidtion in human stratum corneum. Enhancement by dimethylacetamide. demethylsulfoxide. and methylethylether. Arch.Dermatol. 94: 646-648 (1966).
- Suckert, V.R. Die Wirkung von Dimethylsulfozyd auf die Crontronol-arthritis des Kaninchen-kniegelenkes. Buchbesprechungen. 81: 157-158 (1969).
- Sulzberger, M.B., Cortese, T.A., Jr., Fishman, L., Wiley, H.S., and Peyakovich, P.S. Some effects of DMSO on human skin in vivo. Ann N.Y. Acad. Sci. 141: 437-450 (1967).
- Svingen, B.A., Powis, G., Appel, P.L., and Scott, M. Protection against
adriamycininduced skin nectrosis in the rat by dimethyl sulfoxide and
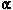 -tocopherol.
Cancer Res. 41: 3395-3399 (1981).
-tocopherol.
Cancer Res. 41: 3395-3399 (1981).
- Tarell, C., Ferrero, D., Gallo, E., Pagliardi, L., and Ruscetti, F.W. Induction of differentiation of HL-60 cells by dimethyl sulfoxide: Evidence for a stochastic model not linked to the cell division cycle. Cancer Res. 42: 445-449 (1982).
- Tersawa, T., Miura, Y., and Masuda, R. The mechanism of the action of DMSO on the heme synthesis of quail embryo yolk sac cells. Exp. Cell Res. 133: 31-37 (1981).
- Teso, D., Morita, A., Bella, A., Jr., Luu, P., and Kim, Y.S. Differential effects of sodium butylate, dimethyl sulfoxide and retinoic acid on membrane-associated antigen, enzymes, and glycoproteins of human rectal adenocarcinoma cells. Cancer Res. 42: 1052-1058 (1982).
- Turco, S.J., and Canada, A.T. Effects of dimethyl sulfoxide in lowering electrical skin resistance. Amer. J. Hosp. Pharm. 26: 120-122 (1969).
- Wieser, P.B., Zeiger, M.A., and Fain, J.N. Effects on dimethyl sulfoxide on cyclic AMP accumulation, lipolysis and glucose metabolism of fat cells. Biochem. Pharmacol. 26: 775-778 (1977).
- Weissman, G., Sessa, G., and Bevans, V. Effect of DMSO on the stabilization of lysosomes by cortisone and chloroquine in vitro. Ann. N.Y. Acad. Sci. 141: 326-332 (1967).
- Zwigeistein, G., Tapiero, H., Portoukalian J., and Fourcade, A. Changes in phospholipid and fatty acid composition in differntiated Friend leukemic cells. Biochem. Biophys. Res. Commun. 98: 349-358 (1981).
Source: Received September 9, 1985. Accepted September 16, 1985 by the Academic Press, Inc. Printed 1985 (pp. 14-27). DMSO Organization wishes to thank the Academic Press, Inc., for allowing us to place this article on our World Wide Web site. Academic Press retains all copyright. To copy any portion of this article, please obtain permission from the publisher.
© 2001-2022 All rights reserved