|
Topical Alpha-Interferon Ointment with Dimethyl
Sulfoxide in the Treatment of Recurrent Genital Herpes
Simplex
J. Shupack, M. Stiller, L Davis, C
Kenny, L. Jondreau
Section of Dermatopharmacology • Department of
Dermatology • New York University Medical Center
New York, N.Y.
Received: February 21, 1991
Abstract
The recent in vivo appearance of acyclovir-resistant
strains of herpes simplex virus stresses the need for
new therapeutic agents to combat this common virus.
Topical interferon preparations may help fill this void.
In the present study dimethyl sulfoxide was combined
with 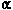 -interferon
in an ointment base to increase percutaneous penetration.
In this double-blind, placebo-controlled trial, patients
with recurrent genital herpes simplex using the topical -interferon
in an ointment base to increase percutaneous penetration.
In this double-blind, placebo-controlled trial, patients
with recurrent genital herpes simplex using the topical
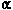 -interferon
preparation had a more rapid cessation of viral shedding
when compared to the placebo group (66% culture negative
on day 1 vs. 25% of placebo patients; p<0.02). In
the 90-day post-treatment period, interferon-treated
patients had fewer recurrences than their placebo-treated
counterparts (1.18 vs. 2.25). This reduction while not
statistically significant was encouraging, (P<0.10). -interferon
preparation had a more rapid cessation of viral shedding
when compared to the placebo group (66% culture negative
on day 1 vs. 25% of placebo patients; p<0.02). In
the 90-day post-treatment period, interferon-treated
patients had fewer recurrences than their placebo-treated
counterparts (1.18 vs. 2.25). This reduction while not
statistically significant was encouraging, (P<0.10).
Interferon, Topical interferon, Herpes simplex virus,
Dimethyl sulfoxide, Acyclovir resistance
Recently, the incidence of genital herpes simplex infections
in the United States has risen dramatically.1 There are an estimated 260,000-500,000 cases per year, and genital herpes simplex virus (HSV) infections are a source of significant physical and psychological morbidity.2
Clinical studies in patients with recurrent genital
herpes simplex have demonstrated a decrease in frequency
and shortened duration of episodes, as well as a more
rapid healing of lesions in those receiving oral acyclovir
versus placebo. Oral acyclovir has
been shown to safely decrease the frequency of episodes
when taken daily for periods up to 3 years.3-10
Straus et al.11 recently reported
that chronic suppressive oral acyclovir failed to reduce
the rate of asymptomatic viral shedding. It is potentially
significant that these investigators cultured acyclovir-resistant
strains of HSV from asymptomatic, nonimmunocompromised
hosts with increased frequency.Interferon,
a glycoprotein with antiviral and antineoplastic activity,
was first shown to be beneficial in preventing reactivation
of herpes labialis in patients undergoing microneurological
decompression of the trigeminal nerve. 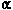 -Interferon
administered intramuscularly prior to operation significantly
reduced viral shedding and new lesion formation in these
patients.12We recently reported that
topical -Interferon
administered intramuscularly prior to operation significantly
reduced viral shedding and new lesion formation in these
patients.12We recently reported that
topical 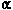 -interferon
(at a concentration of 106 IU/g in a nonoxynol-9
gel base) was more effective than placebo in a double-blind,
placebo-controlled clinical trial in patients with recurrent
genital HSV. (Nonoxynol-9 is a widely used, safe surfactant
which aids in the uniform dispersion of interferon in
the vehicle.13 The duration of viral
shedding and the time until the end of all subjective
symptoms including pain, burning and itching were significantly
reduced in patients receiving the interferon preparation.14
Friedman-Kein et al.15 using
a similar topical -interferon
(at a concentration of 106 IU/g in a nonoxynol-9
gel base) was more effective than placebo in a double-blind,
placebo-controlled clinical trial in patients with recurrent
genital HSV. (Nonoxynol-9 is a widely used, safe surfactant
which aids in the uniform dispersion of interferon in
the vehicle.13 The duration of viral
shedding and the time until the end of all subjective
symptoms including pain, burning and itching were significantly
reduced in patients receiving the interferon preparation.14
Friedman-Kein et al.15 using
a similar topical 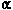 -interferon
preparation reported favorable results. In their placebo-controlled
study there was a decrease in viral shedding, time to
scabbing and formation of new lesions in the group of
patients who received the -interferon
preparation reported favorable results. In their placebo-controlled
study there was a decrease in viral shedding, time to
scabbing and formation of new lesions in the group of
patients who received the 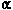 -interferon.
Several clinical trials by Israeli investigators have
evaluated topical human fibroblast ( -interferon.
Several clinical trials by Israeli investigators have
evaluated topical human fibroblast (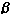 )-interferon
in the treatment of recurrent genital and facial HSV
infections. These investigators reported not only a
decrease in the duration of episodes and severity of
symptoms but also a decrease in the subsequent recurrence
rate.16-18 Topical acyclovir in an
aquaeous propylene glycol cream base has shown significant
efficacy in the treatment of recurrent genital HSV infections
in controlled clinical trials.19-22
On the other hand, 5% acyclovir in a polyethylene glycol
ointment base, the topical preparation which is commercially
available in the United States, has failed to show significant
clinical efficacy in double-blind studies.23-26
Furthermore, topical acyclovir use has failed to alter
the frequency of recurrences or the amount of time between
recurrences.27-28In the present study )-interferon
in the treatment of recurrent genital and facial HSV
infections. These investigators reported not only a
decrease in the duration of episodes and severity of
symptoms but also a decrease in the subsequent recurrence
rate.16-18 Topical acyclovir in an
aquaeous propylene glycol cream base has shown significant
efficacy in the treatment of recurrent genital HSV infections
in controlled clinical trials.19-22
On the other hand, 5% acyclovir in a polyethylene glycol
ointment base, the topical preparation which is commercially
available in the United States, has failed to show significant
clinical efficacy in double-blind studies.23-26
Furthermore, topical acyclovir use has failed to alter
the frequency of recurrences or the amount of time between
recurrences.27-28In the present study
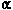 -interferon
was combined with dimethyl sulfoxide (DMSO). DMSO, a
dipolar aprotic solvent, has been
shown in clinical studies to be effective in enhancing
the penetration of topical medications.29-31We
report the results of a study comparing the efficacy
and safety of a topical -interferon
was combined with dimethyl sulfoxide (DMSO). DMSO, a
dipolar aprotic solvent, has been
shown in clinical studies to be effective in enhancing
the penetration of topical medications.29-31We
report the results of a study comparing the efficacy
and safety of a topical 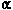 -interferon
ointment which also contained DMSO in the treatment
of recurrent genital herpes. -interferon
ointment which also contained DMSO in the treatment
of recurrent genital herpes.
Materials
and Methods
The study population consisted of 40 immunocompetent
men and women, aged 18 years or older, who had had at
least two episodes of recurrent genital herpes simplex
within the past 6 months. Diagnosis was confirmed by
viral culture or by a dermatologist's clinical evaluation.
All patients agreed to discontinue the use of topical
and systemic medications intended for treatment of herpes
simplex or which might alter symptoms such as pruritus
or pain, e.g. antihistamines and analgesics,
30 days prior to enrollment and for the duration of
the study. A phisical examination and medical history
were obtained on all subjects prior to enrollment. This
clinical trial was approved by the Institutional Board
of Research Associates of New York University Medical
Center prior to its initiation, and an informed consent
was obtained from all participants in accordance with
Food and Drug Administration guidelines. This clinical
trial was double-blinded with a placebo control. The
patients were given identically labeled tubes of either
105 IU/g 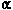 -interferon
in hydrophilic ointment with 5% DMSO or hydrophilic
ointment with 5% DMSO, which served as a placebo control.
The decision to use a lower concentration of interferon
than was used in our earlier clinical trial 14 was based on consideration of the more penetrating DMSO-containing
vehicle in the present study. Human leukocyte interferon
was prepared by Vira-Tech Inc. The ointment base contained
water, petrolatum, mineral oil, mineral wax, wool alcohol,
2-bromo-2-nitropropane-1,3-diol, benzyl alcohol, carboxymethyl
cellulose and 5% DMSO.The patients were interviewed
at a time when they were not having a herpetic outbreak
and instructed to begin applying the ointment four times
a day at the onset of the first prodromal symptoms of
their next attack. All patients were then examined at
our institution within 24 h of the onset of that
episode of herpes simplex (day 1) and again on
days 3, 5, and 7. Outbreaks were treated until resolution
or up to a maximum of 7 days. Subsequent recurrences
were not treated. Additionally, all participants were
required to make a follow-up visit to NYU Medical Center
60-90 days after the day 5 visit. Additionally, during
the 6-month period after cessation of therapy, the study
subjects were expected to keep a diary recording the
number of herpetic episodes they had and the duration
of those recurrences. This information was obtained
from the study subjects in a 6-month post-treatment
phone interview conducted by one of the study coordinators.
During all visits herpetic lesions were morphologically
staged (as papules, vesicles, crusts, or healed), lesion
counts and measurements taken, and patients were asked
to rate their subjective symptoms (which included pain,
pruritus, burning, and stinging). Patient serum was
collected on day 3 to determine serum electrolytes,
liver function tests, hematologic profiles as well as
DMSO levels. DMSO serum levels were measured by gas
chromatography at the National Medical Services Laboratory,
Willow Grove, Pa., USA. Specimens for virus isolation
were taken on days 1, 3, and 5 by the methods used by
Friedman-Klein et al.15Statistics
were prepared by -interferon
in hydrophilic ointment with 5% DMSO or hydrophilic
ointment with 5% DMSO, which served as a placebo control.
The decision to use a lower concentration of interferon
than was used in our earlier clinical trial 14 was based on consideration of the more penetrating DMSO-containing
vehicle in the present study. Human leukocyte interferon
was prepared by Vira-Tech Inc. The ointment base contained
water, petrolatum, mineral oil, mineral wax, wool alcohol,
2-bromo-2-nitropropane-1,3-diol, benzyl alcohol, carboxymethyl
cellulose and 5% DMSO.The patients were interviewed
at a time when they were not having a herpetic outbreak
and instructed to begin applying the ointment four times
a day at the onset of the first prodromal symptoms of
their next attack. All patients were then examined at
our institution within 24 h of the onset of that
episode of herpes simplex (day 1) and again on
days 3, 5, and 7. Outbreaks were treated until resolution
or up to a maximum of 7 days. Subsequent recurrences
were not treated. Additionally, all participants were
required to make a follow-up visit to NYU Medical Center
60-90 days after the day 5 visit. Additionally, during
the 6-month period after cessation of therapy, the study
subjects were expected to keep a diary recording the
number of herpetic episodes they had and the duration
of those recurrences. This information was obtained
from the study subjects in a 6-month post-treatment
phone interview conducted by one of the study coordinators.
During all visits herpetic lesions were morphologically
staged (as papules, vesicles, crusts, or healed), lesion
counts and measurements taken, and patients were asked
to rate their subjective symptoms (which included pain,
pruritus, burning, and stinging). Patient serum was
collected on day 3 to determine serum electrolytes,
liver function tests, hematologic profiles as well as
DMSO levels. DMSO serum levels were measured by gas
chromatography at the National Medical Services Laboratory,
Willow Grove, Pa., USA. Specimens for virus isolation
were taken on days 1, 3, and 5 by the methods used by
Friedman-Klein et al.15Statistics
were prepared by 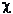 2
analysis and Student's t test where applicable. 2
analysis and Student's t test where applicable.
Results
Of the 40 patients who initially enrolled in this patient-initiated
clinical trial and received tubes of medication, 20
completed therapy of one HSV recurrence (12 medicated,
8 placebo). Of these 20, 19 individuals continued participation
in the study through the 60- to 90-day posttreatment
follow-up visit.The patients in the interferon-treated
group demonstrated decreased viral shedding and more
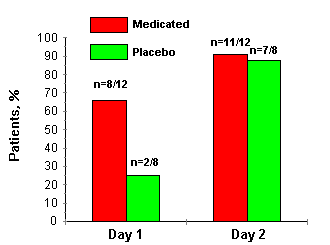
rapid healing than those in the placebo group. 66% of
the interferon-treated patients were culture negative
after 24 h of treatment (day 1), whereas only 25%
of the patients in the placebo group were culture negative
at that time (p<0.02). By day 5, 91% of the interferon
group was culture negative compared to 85% of the placebo
group (p > 0.66; fig. 1).
On day 5, 25% of the patients in the placebo group had
active lesions, while no patients in the interferon
group had active lesions (p > 0.02).
Figure
1.
Patients
presenting with a negative viral isolation text.
After completion of therapy, the patients in the placebo
group had twice as many recurrences over the ensuing
6 months as did their counterparts who had received
interferon ointment. Furthermore, the recurrences in
the placebo-treated group took 2.3 days longer to heal
than recurrences in the interferon group (p>0.10; table 1). At the end of the study,
the patients were asked whether or not they believed
that their treatment had been useful. Eleven of the
12 patients (92%) in the interferon group rated their
treatment as 'useful.' Only 1 of 8 patients (12%) in
the placebo group regarded treatment as 'useful' (p<0.003).
The physician's global evaluation corresponded rather
closely with that of the patients. Investigators rated
therapy as worthwhile in 11/12 (92%) of individuals
in the interferon group versus 2/8 (25%) in the placebo
group (p<0.01).
Table
1.
Average
number of recurrences (after treatment)
| |
Medicated |
Placebo |
| Mean |
1.18 |
2.25 |
| Sd |
0.83 |
1.39 |
| Number |
11 |
8 |
| Minimum |
0 |
0 |
| Maximum |
2 |
5 |
| t |
-1.93 |
|
| d.f. |
17 |
|
| p |
<
0.10 |
|
| Median |
1 |
2 |
Discussion
These results further support our belief that topical
interferon preparations mav have a role in the treatment
of recurrent HSV infections.13-18
Topical interferon could be used in place of systemic
acyclovir in patients who dislike taking oral medications.
Additionally, a topical agent, such as the one evaluated
in this study or our earlier clinical trial,12
could be used as an adjunct along with systemic acyclovir
to minimize the emergence of acyclovir-resistance strains
of HSV.8,32-43One
area of concern is the high dropout rate in this clinical
trial. Only 20/40 study subjects completed therapy of
one recurrent episode. This 50% dropout rate is not
unusually high for a patients-initiated study for recurrent
herpes. In 1983, Fiddian et al.19
reported that 62% of the 137 patients enrolled in their
acyclovir cream study for recurrent genital HSV completed
that clinical trial. In a larger multicenter study evaluating
acyclovir ointment in recurrent genital herpes, 56%
of the 547 entering patients completed therapy.23
In another multicenter clinical trial testing topical
acyclovir in recurrent oral HSV, Spruance et al.44
reported that only 20% of the 352 enrollees finished
the study. Subsequently, our 50% dropout rate does not
seem alarmingly high for a patient-initiated study of
this type.Based upon published studies evaluating a
variety of topical interferon preparations in recurrent
facial and Genital HSV infections, it is not possible
to select an optimal preparation. The topical 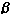 -interferon
cream tested by Israeli investigators16-18
and the topical -interferon
cream tested by Israeli investigators16-18
and the topical 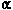 -interferon
preparations evaluated by us and Friedman-Kien et
al.14.15 were all similarly impressive
in reducing the duration of viral shedding and of subjective
symptoms. In the present clinical trial and in the study
by Glezerman et al.,6 there
was an impressive decrease in the recurrence rate and
in the seventy of subsequent recurrences after the study
terminated and use of topical interferon was discontinued.
This can be just as easily attributed to the small study
population in both clinical trials (40 patients in the
present study and 25 in the report of Glezerman et
al.) as to any long-term, positive residual pharmacologic
effect from interferon.As described under Materials
and Methods, the preparation tested contained DMSO.
This was added to enhance the penetration of interferon.19-21
Although we cannot unequivocally exclude the possibility
that DMSO enhances the antiviral activity of interferon,
we can confidently assert that DMSO absorption was not
significant and that there was no systemic toxicity
(as shown by DMSO drug levels, blood chemistries, hematologic
profiles, and physical examination).In summary, we feel
that topical -interferon
preparations evaluated by us and Friedman-Kien et
al.14.15 were all similarly impressive
in reducing the duration of viral shedding and of subjective
symptoms. In the present clinical trial and in the study
by Glezerman et al.,6 there
was an impressive decrease in the recurrence rate and
in the seventy of subsequent recurrences after the study
terminated and use of topical interferon was discontinued.
This can be just as easily attributed to the small study
population in both clinical trials (40 patients in the
present study and 25 in the report of Glezerman et
al.) as to any long-term, positive residual pharmacologic
effect from interferon.As described under Materials
and Methods, the preparation tested contained DMSO.
This was added to enhance the penetration of interferon.19-21
Although we cannot unequivocally exclude the possibility
that DMSO enhances the antiviral activity of interferon,
we can confidently assert that DMSO absorption was not
significant and that there was no systemic toxicity
(as shown by DMSO drug levels, blood chemistries, hematologic
profiles, and physical examination).In summary, we feel
that topical 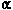 -interferon
is a potentially safe and useful therapy for recurrent
herpes genitalis. It might be utilized adjunctively
along with oral acyclovir or as monotherapy, either
during recurrences or by asymptomatic carriers between
episodes to diminish the emergence of resistant strains
of HSV. -interferon
is a potentially safe and useful therapy for recurrent
herpes genitalis. It might be utilized adjunctively
along with oral acyclovir or as monotherapy, either
during recurrences or by asymptomatic carriers between
episodes to diminish the emergence of resistant strains
of HSV.
References
- Gonzolez E.: Sexually transmitted
diseases: in Fitzpatrick T.B., Eisen A.Z.. Wolff K.,
Freedberg I.M., Austen K.F. (eds): Dermatology
in General Medicine. New York, McGraw-Hill, 1987,
vol. 2, pp 2385-2467.
- Burnett J.W., Crutcher W.A.: Viral
and rickettsial infections: in Moschella S.L. Hurley
H.J. (eds): Dermatology. Philadelphia. Saunders.
1985, vol 1. pp 681-683.
- Mindel A. Faherty A. Hindley D. et
al: Prophylactic oral acyclovir in recurrent genital
herpers. Lancet 1984:ii:57-59.
- Fiddian A.P., Halsos A.M., Kinge
B.R., et al: Oral acyclovir in the treatment
of genital herpes. Proceedings of a symposium on acyclovir
sponsored by Burroughs Welcome. Am J Med 1982;73;335-337.
- Straus S.E., Takiff H.E., Seidlin
M.: Suppression of frequently recurring genital herpes:
A placebo-controlled double-blind trial of oral acyclovir.
N Engl J Med 1984:310:1545-1550.
- Bryson Y.J., Dillon M., Lovett M.:
Treatment of first episodes of genital herpes simplex
virus infection with oral acyclovir. N Eng J Med
1983:308:916-921.
- Douglas J.M., Critchlow M.S., Benedetti
J.: A double-blind study of oral acyclovir for suppression
of recurrences of genital herpes simplex virus infection.
N Engl Med 1984:310:1551-1556.
- Straus S.E., Cruen K.D., Sawyer M.H.,
et al: Acyclovir suppression of frequently
recurring genital herpes. Efficacy and diminishing
need during successive years of treatment. JAMA 1988:260:2227-2230.
- Mertz G.J., Lawrence E., Kaufman
R., et al: Prolonged continuous versus intermittent
oral acyclovir treatment in normal adults with frequently
recurring genital herpes simplex virus infection.
Am J Med 1988:85:14-19.
- Kaplowitz L.G., Baker D., Gelb L.,
et al: Prolonged continuous acyclovir treatment
of normal adults with frequently recurring genital
herpes simplex vius infection. Am J Med 1988:85:14-19.
- Straus S.E., Mindell S., Takiff
H.E., et al: Effect of oral acyclovir treatment
on symptomatic and asymptomatic virus shedding in
recurrent genital herpes. Sex Transm Dis 1989:16:107-112.
- Pazin G.J., Armstrong J.A., Lam
M.T.: Prevention of reactivated herpes simplex infection
by human leukocyte interferon after operation on the
trigeminal rooot. N Engl J Med 1979:301:225-230.
- Asculai S.S., Weis M.T., Rancourt
M.W., et al: Inactivation of heres simplex
viruses by nonionic surfactants. Antimicrob Agents
Chemother 1978:13-686-690.
- Shupack J., Stiller M., Knobler
E., Ackerman C., Jondreau L., Kenny C.: Topical alpha-infection.
A double-blind, placebo-controlled clinical trial.
Dermatologica 1990:181:134-138.
- Friedman-Kein A.E., Klein R.J.,
Glaser R.D.: Treatment of recurrent genital herpes
with topical alpha interferon get combined with non-oxynol-9.
J Am Acad Dermatol 1986:15:989-994.
- Glezerman M., Cohen V., Movshovitz
M., et al: Placebo controlled trial of topical
interferon in labial and genital herpes. Lancet 1988:i:150-152.
- Movshovitz M., Schwach-Millet M.,
Kriss-Leventon S., et al: Topical treatment
of recurrent facial and genital herpes with human
interferon
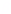 cream; in Kono R. (ed): Herpes Virus Chemotherapy.
Amsterdam. Elsevier Science Publishers. 1985, pp.
285-292.
cream; in Kono R. (ed): Herpes Virus Chemotherapy.
Amsterdam. Elsevier Science Publishers. 1985, pp.
285-292.
- Isacsohn M., Berson B., Sternberg
I., Murag A.: Human fibroblast interferon treatment
of viral diseases of the skin and mucous membranes.
Isr J Med Sci 1983:19:959-962.
- Fiddian A.P., Kinghorn G.R., Goldmeier
D., et al: Topical acyclovir in the treatment
of genital herpes: A comparison with systemic therapy.
J Antimicrob chemother 1983:3:291-301.
- Kinghorn G.R., Turner E.B., Barton
I.G., et al: Efficacy of topical acyclovir
cream in first and recurrent episodes of genital herpes.
Antiviral Res 1983:3:291-301.
- Fiddian A.P., Brigden D., Yeo J.M.,
et al: Acyclovir: An update of the clinical
applications of this antiherpes agent. Antiviral
Res 1984:4:99-117.
- Kinghorn G.R.: Topical acyclovir
in the treatment of recurrent herpes simplex virus
infections. Scand J Infect Dis 1985:suppl 47:58-62.
- Luby J.P., Gnann J.W., Alexander
J.W., et al: A collaborative study of patient-initiated
treatment of recurrent genital herpes with topical
acyclovir or placebo. J Infect Dis 1984:150:1-6.
- Reichman J., Badger G.J., Guinan
M.E., et al: Topically administered acyclovir
in the treatment of recurrent herpes simplex genitalis:
A controlled trial. J Infect Dis 1983:147:336-340.
- Corey L., Nahmias A.J., Guinan M.E.,
et al: A trial of topical acyclovir in genital
herpes simplex N Engl J Med 1982:306:1313-1319.
- Freeman D.J., Sheth N.V., Spruance
S.L.: Failure of topical acyclovir in ointment to
penetrate human skin. Antimicrob Agents Chemother
1986:29:730-732.
- Barton I.G., Kinghorn G.R., Rowland
M., et al: Recurrences after first episodes
of genital herpes in patients treated with topical
acyclovir cream. Antiviral Res 1984:4:293-300.
- Fawcett H.A., Wansbrough-Jones M.H.,
Clark A.E., et al: Prophylactic topical acyclovir
for frequent recurrent herpes simplex infection with
and without erythema multiforme. Br Med J 1983:287:798-799.
- Morrison R.T., Boyd R.H.: Organic
Chemistry. Newton, Allyn, and Bacon. 1983. pp.
33-34.
- Fisher A.A.: Dimethyl sulfoxide
as a vehicle for food allergy patch tests. Cutis
1986:42:109-110.
- Goldman L., Igelman J.M., Kitzmiller
K.: Investigative studies with DMSO in dermatology.
Ann NY Acad Sci 1967:141:429-436.
- Erlich S.K., Mills J., Chatis P.,
et al: Acyclovir resistant herpes simplex virus
infections in patients with the acquired immunodeficiency
syndrome. N Engl J Med 1988:320:293-296.
- Chatis P.A., Miller C.H. Schrager
L.E. Crumpacker C.S.: Successful treatment with foscarnet
of an acyclovir resistant mucocutaneous infection
with herpes simplex virus in a patient with acquired
immunodeficiency syndrome. N Engl J Med 1989.320:297-300.
- Burns W.H., Santos G.W., Soral R.,
et al: Isolation and characterization of resistant
herpes simplex virus after acyclovir therapy. Lancet
1982:i:421-423.
- Wade J.C., McLaren C., Myers J.D.:
Frequency and significance of acyclovir-resistant
herpes simpiex virus isolated from marrow transplant
patients receiving multiple courses of treatment with
acyclovir. J Infect Dis 1983:148:1077-1082.
- Schinazi R.F., del Bene V., Scott
R.T., Dudley-Thorpe J.B.: Characterization of acyclovir-resistant
and sensitive herpes simplex viruses from a patient
with an acquired immune deficiency. J Antimicrob
Chemother 1986,18(suppl):127-134.
- Swennerholm B., Vohline A., Lowhogen
G.B., et al: Sensitivity of HSV strains isolated
before and after treatment with acyclovir. Scand
J Infect Dis 985:47:149-154.
- Crumpacker C.S., Schnipper L.E.,
Marlowe S.I., Kowalsky P.N., Hershey B.J., Levin M.J.:
Resistance to antiviral drugs of herpes simplex virus
isolated from a patient treated with acyclovir. N
Engl J Med 1982:306:343-346.
- Crumpacker C: Significance of resistance
of herpes simplex virus to acyclovir. J Am Acad
Dermatol 1988;18:190-195.
- Ellis M.N., Keller P.M., Fyfe J.A.,
et al: Clinical isolate of herpes simplex type
2 that induces a thymidine kinase with altered substrate
specificity. Antimicrob Agents Chemother 1987;31:1117-1125.
- Dekker C., Ellis M.N., McLaren C.,
et al: Virus resistance in clinical practice.
J Antimicrob Chemother 1983;12(suppl):137-152.
- Burns W.E., Santos G.W., Soral R.,
et al: Isolation and characterization of resistant
herpes simplex virus after acyclovir therapy. Lancet
1982:i:421-423.
- Wade J.C., McLaren C., Myers J.D.:
Frequency and significance of acyclovir-resistant
herpes simplex virus isolated from marrow transplant
patients receiving multiple courses of treatment with
acyclovir. J Infect Dis 1983.148:1077-1082.
- Spruance S.L., Crumpacker C.S., Schnipper
L.E., et al: Early, patient-initiated treatment
of herpes labialis with topical 10% acyclovir. Antimicrob
Agents Chemother 1984.23:553-555.
Source
Pharmacology and Treatment, Dermatology 1992;184:40-44
This clinical trial was supported by a grant from Virapen
Corporation.
Jerome Shupack, MD
Department of Dermatology
New York University Medical Center
562 First Avenue, New York, NY 10016 (USA)© 1992
Karger AG. Base
1018-8665/92/1841-0040
$ 2.75
DMSO Organization thanks the Dermotology publishers
for allowing this article to be placed on our World
Wide Web Site. The publisher retains all copyright.
Please contact the publisher for permission to copy
any portion of this article.
|


